Introduction
•Evaluation of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) is routinely done on breast cancer tissue specimens .
•These markers are essential for treatment decision making (ie hormonal treatment for ER/PR+ and trastuzumab for HER2+). Due to expense of treatment and side effects/toxicity, those who are negative should not be treated with adjuvant therapy.
•Both the ASCO/CAP and NCCN recommendations are based on immunohistochemical (IHC) results on tissue sections fixed in 10% neutral-buffered formalin for at least 6–8 hours
•While fine-needle aspiration is no longer a widely used modality for primary breast cancer diagnosis, it is still used for recurrent and metastatic breast tumors, and in effusion cytology.
•Cytologicpreparations often use alternative fixatives, such as alcohol-based fixatives, rather than more hazardous fixatives like formalin; however, alcohol based fixatives coagulate tissue proteins and thus may affect the epitope retrieval process and result in less reliable IHC staining
•The authors’ previous study showed that alcohol fixation did not affect ER results when alcohol fixed cell block (CB) sections were compared to formalin fixed tissue samples, while PR and HER2 IHC results were less concordant with formalin fixedsurgical samples. The aim of this study is to evaluate if ER, PR, and Her2 IHC results on formalin-fixed CB sections from FNAs correlate better than alcohol-fixed samples to those observed on surgical specimens (SS)
Findings
•50 cases of formalin fixed CB samples obtained from 9 primary and 41 metastatic breast carcinomas were studied, and all of these patients had corresponding SS from the primary breast tumor. ER, PR, and Her2 IHC were done on all samples
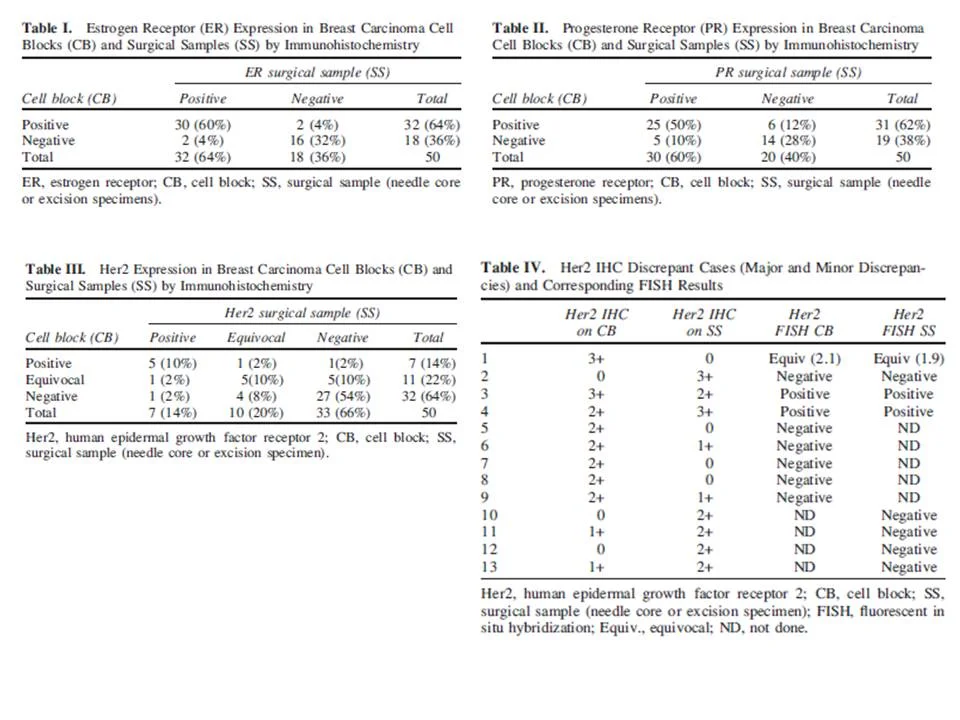
•ER results (Table 1) were in complete agreement in 46 cases (92%, cc=0.82, P<0.0001)
•PR results (Table 2) were in complete agreement in 39 cases (78%, cc=0.433, P=0.0021)
•Her2 results (Tables 3 and 4) were in complete agreement in 37 cases (74%, cc=0.439, P=0.0020). Of the 13 discrepancies, 2 cases were“major” discrepancies (Her2 was 3+ on one sample and negative (0, 1+) on the other and 11 cases were “minor” discrepancies (one sample was negative or equivocal, and the other was equivocal or positive).
-The two cases with major discrepancies in Her2 IHC results (Table 4, cases 1 and 2) showed equivocal result for Her2 amplification by FISH in one case and a negative result in the other.
Conclusions
•Although the ASCO/CAP and NCCN guidelines were primarily established on needle core and excision specimens, this study showed that ER IHC results on CB samples demonstrate good to excellent agreement with the corresponding surgical specimens,
•This study suggests that alcohol fixation does not have significant affect on ER detection, and the use of formalin fixation for CB preparations does not significantly improve the detection of ER positive breast tumors
•There was 78% positive agreement in PR results between CB and SS. The use of 10% neutral formalin as a CB fixative resulted in increase in PR concordance rate to cc =0.433 (from 0.38 in ethanol-fixed samples (authors’ previous study). While the positive agreement in PR results is still less than in ER, this improvement suggests that PR detection may be affected by alcohol fixation.
•Formalin fixation of CB samples does not improve the detection of Her2 overexpression (cc =0.439 compared with 0.45 in their previous study). The number of equivocal Her2 IHC results is comparable between CB and SS. However, the discrepancy rate between CB and SS remains significant (13 cases, 26% in this study). Therefore, they conclude that alcohol fixation alone can not be blamed for discrepant Her 2 IHC results on CB versus SS samples., and further studies are needed to address other possible confounders
Introduction
• Myoepithelial cells (MECs) surround the mammary ductal-lobular system
• They have roles in normal breast development and physiology, as well as functioning as tumor suppressors by functioning as a physical barrier through their maintenance of basement membrane around the ducts and lobules
• Studies had shown that MECs surrounding the spaces involved by ductal carcinoma in situ demonstrated important molecular, genetic, and epigenetic differences from those that lined normal ducts and lobules, and thus speculation that they were key regulators in the progression of DCIS to invasive breast carcinoma
• As part surgical pathology practice, immunohistochemistry can be utilized in problematic cases to distinguish both in situ and sclerosing lesions from invasive carcinoma
• Myoepithelial markers that can be utilized by IHC include smooth muscle actin, calponin, smooth muscle myosin heavy chain, p63, CD10, cytokeratin 5/6, and p75
• Recent studies had shown that while the MECs spaces involved by DCIS are retained, the immunophenotype differed from those of MECs surrounding normal ducts and lobules. In particular, SMMHC expression was reduced in 3/4 of cases, and CK5/6 and CD10 in 1/3
• Although the biologic implication of these alterations is unknown, the practical considerations is apparent: Reduced staining with myoepithelial markers could produces a “false negative” result where the lack of staining around some groups of DCIS is erroneously categorized as invasive
• With this in mind, the purpose of this paper was to evaluate the immunophenotype of benign sclerosing lesion associated MECs
Findings
• Included in this study were 48 cases of benign sclerosing lesions (sclerosing adenosis, radial scars, and complex sclerosing lesions)
• Sections were then stained for the 7 MEC markers listed above
• Staining strength was scored in relation to adjacent benign ducts and lobules (which were assigned a score of 3): MECs in sclerosing lesions that had the same staining as adjacent benign ducts and lobules were given a score of 3; those with slightly less staining were given a score of 2; those with markedly decreased were given a score of 1; and those with no staining were given a score of 0
• They found that staining intensity was reduced in at least 1 MEC marker in 23 of 48 cases (47.9%). Two or more markers were reduced in 11 of 48 cases (22.9%).
• Table 2 shows the number of cases with reduced staining according to each MEC marker. The most striking difference was the decrease in staining intensity for CK5/6, with 31.8% of cases showing decreased intensity staining for it and 15.9% showing complete absence of it
• Staining intensity was also compared according to type of benign sclerosing lesion (sclerosing adenosis versus radial scars/complex sclerosing lesions), seen in Table 3. MEC staining for CD10 and p63 was significantly decreased in radial scars/complex sclerosing lesions as compared to sclerosing adenosis (26.9% vs 0%, and 17.4% vs 0%, respectively)
• Compared to the authors’ previous study on DCIS and MECs, they had found in that study that 84% of DCIS cases showed decreased or absent MEC markers, and in particular, 76.5% of cases showed decrease in SMMHC. Thus, the alterations in sclerosing lesions are less frequent when compared to DCIS, and also are different with regard to which MEC marker showed reduced expression
Conclusions
• While it had been shown that the myoepithelial cells that surround spaces involved by ductal carcinoma in situ show phenotypic differences from normal myoepithelial cells, the immunophenotype of myoepithelial cells that surround the entrapped glandular spaces in benign sclerosing lesions had not yet been characterized
• The results of this study indicate that the myoepithelial cells associated with benign sclerosing lesions of the breast may show immunophenotypic differences from those surrounding normal mammary ducts and lobules
• Thus, it shouldn’t be assumed that an antibody that is sensitive for the detection of MECs in normal breast tissue will be equally sensitive for their detection in pathologic conditions, and markers may not be equally sensitive in different pathology conditions. Moreover, it shouldn’t be concluded that MECs are absent based on lack of staining for 1 MEC marker, and thus a panel should be used
• It is important for pathologists to keep these findings in mind when considering which MEC markers to use in the distinction of benign sclerosing lesions from invasive carcinoma in order to help prevent erroneously categorizing a benign lesion from invasive carcinoma
Introduction
•GATA3-binding protein is a transcription factor of the GATA family, nuclear proteins that recognize G-A-T-A nucleotide sequences in target gene promoters and activate or repress those genes
•GATA3 is important in the development and function of of breast epithelia, urothelial, and subsets of T lymphocytes. Other known functions include gene regulation in development/maintenance of skin, esp hair shafts, trophoblast, and some endothelial cells
•Studies of GATA3 had shown that most primary and metastatic mammary carcinomas express GATA3 (80-90%), although less so in triple negative tumors (67%), and that it seemed to have higher expression in lower-grade tumors
•Another study had shown strong expression of GATA3 detected only in mammary (94%), urothelial (86%), and endometrial (2%) adenocarcinomas, but not in pulmonary, pancreatic, colonic, prostatic, and ovarian carcinomas in their small sample set
•This study immunohistochemically examined 2500 epithelial and mesenchymal or neuroectodermal tumors for GATA3 to further explore its specificity for mammary and urothelial carcinomas
Findings
•2040 epithelial neoplasms and 460 mesenchymal tumors were examined, as well as a spectrum of normal adult, fetal, and embryonic tissue samples. Lymphomas were excluded from the study
•The results of GATA 3 expression in epithelial neoplasms is summarized in Table 1
•The majority of primary breast ductal carcinomas (163/178, 92%) and their metastases (49/51, 96%) were positive
•Of the negative cases, all but two were high grade ductal carcinomas, and all but 1 were also estrogen receptor negative
•Overall, 30 of the 36 ER negative ductal were GATA3 positive, but they showed heterogeneous, reduced GATA3 expression
•Of 37 metastatic ductal carcinomas tested for both GATA3 and GCDFP-15, GATA3 was more sensitive (92% vs 78%)
•All lobular breast carcinomas (31/31) were positive for GATA3, although 4/10 mucinous carcinomas showed reduced positivity
•Most urothelial carcinomas (49/54) were extensively GATA3 positive, except 5 which were poorly differentiated or showed extensive squamous differentiation. The negative cases all were also negative for thrombomodulin. It should be noted that GATA3 is also expressed in seminal vesicle epithelial
•Cutaneous squamous cell carcinomas were usually positive (81%). Squamous cell carcinomas from other sites, including cervical (33% positive for GATA), laryngeal (16%), and pulmonary (12%) carcinomas were less commonly positive
•Of the other skin tumors, nearly all basal cell carcinomas were strongly and uniformly positive, as were trichoepitheliomas, basaloid cells of pilomatricoma, epithelia of pilar cyst, and seborrheic keratosis
•Salivary gland and pancreatic ductal adenocarcinomas were the most commonly GATA3-positive nonmammary adenocarcinomas (43% and 37% of cases positive)
•Among renal carcinomas, chromophobe carcinomas showed a high frequency (51%) of GATA3 positivity. Oncocytomas were less commonly positive (17%)
•Over half (58%) of malignant mesotheliomas showed nuclear GATA3 positivity, and was seen in both epithelial and sarcomatoid mesotheliomas
•In germ cell tumors, pure choriocarcinomas and choriocarcinoma components of mixed germ cell tumors that were also shown to be positive for b-HCG showed GATA3 positivity
•GATA3 positivity was rare in adenocarcinomas of the lung (6/71)
•The frequency of GATA3 positivity in carcinomas of stomach, colon, prostate, endometrium, ovary, and thyroid did not exceed 7%. Of note, all gastric signet ring cell carcinomas were negative.
•All epithelial neuroendocrine tumors including low grade tumors, such as carcinoids and well-differentiated pancreatic neuroendocrine tumors, and high-grade neuroendocrine carcinomas, such as pulmonary small cell and Merkel cell carcinomas, were negative
•Regarding mesenchymal or neuroectodermal tumors, see Table 2
•GATA3 positivity was detected in pheochromocytoma and extra-adrenal paraganglioma
Conclusions
•Despite its complex distribution in epithelial tumors, GATA3 is potentially useful in several specific settings
•This marker is sensitive, although not totally specific, for mammary and urothelial carcinomas.
•GATA3 is frequently expressed in breast carcinomas and is a potential marker for mammary origin of metastatic carcinoma because metastases show a high percentage of retention for GATA3 expression (Table 3 shows discriminatory value of GATA3)
•Other commonly GATA3-positive epithelial tumors include basal cell carcinoma, cutaneous squamous cell carcinoma, skin adnexal tumors, choriocarcinoma, endodermal sinus tumor, renal chromophobe carcinoma, malignant mesothelioma, and salivary gland and pancreatic ductal adenocarcinoma
•However, GATA3 is rarely expressed in neuroectodermal and mesenchymal tumors, except in pheochromocytoma/paraganglioma, which helps distinguish the latter from neuroendocrine carcinomas (which are negative)